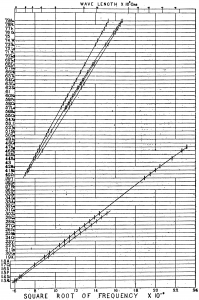
The original Moseley Plot, showing the energies of X-rays from different elements as a function of their atomic number [1].
X-ray fluorescence
When an inner shell electron is ejected from an atom—for example through interaction with an X-ray or high energy electron—the resultant hole left in the shell will be filled by an electron relaxing down from a higher energy state. This electron must lose an amount of energy equal to the difference in energy levels, and it may do this through the emission of an X-ray. Measuring the energy of this X-ray will therefore tell us precisely which energy levels and what species of atom were involved, and this gives us a tool to identify (and even quantify) the elements present in a sample.
Electron beam micro-analysis
The high energy (kilo-electron volt range) electron beam of an electron microscope is one way of exciting these characteristic X-rays from a material. It is possible to mount the required detectors in either a scanning or transmission electron microscope (SEM or TEM), or to use a dedicated instrument, the electron probe micro-analyser (EPMA). Two different methods of detection are possible, each measuring the same X-rays but using quite different principles.
Wavelength-dispersive X-ray spectroscopy
Wavelength-dispersive X-ray spectrometers (WDX or WDS) use a diffracting crystal to disperse the emitted X-rays to different angles depending on their energy. The positions of the crystal and detector are mechanically moved to select the X-ray energy to be measured, while keeping the angle of emergence of the X-ray constant and keeping the sample, crystal and detector on the circumference of a Rowland circle of constant radius. A gas proportional counter is used for detecting the X-rays, and a spectrum is acquired one energy channel at a time by mechanically stepping the diffraction angle.
Energy-dispersive X-ray spectroscopy
Energy-dispersive X-ray spectrometers (EDX or EDS) use a single silicon detector to detect a range of X-ray energies without requiring a dispersive element. As the incoming X-ray is absorbed by the silicon, a number of electron-hole pairs is generated which is proportional to the energy of the X-ray. The electrons and holes are swept to opposite sides of the silicon by an applied electric field, and are read out of the device as a pulse of charge. Discriminating and digitising electronics interpret the magnitude of this charge pulse as an X-ray energy, and a multichannel analyser counts these X-rays and builds up an energy spectrum pulse-by-pulse.
References
[Bibtex]
@Article{Moseley1914PM27,
author = {H. G. J. Moseley},
title = {The high-frequency spectra of the elements. Part II.},
journal = {Philosophical Magazine},
year = {1914},
volume = {27},
pages = {703-713},
doi = {10.1080/14786440408635141}
}